- Product Details
Vietnam Rare Earth Metal Mine Owner Large Supply Available
MONTHLY 50K Ton
About Rare Earth Metals
Rare earth elements are a group of metals that are critical ingredients for a greener economy, and the location of the reserves for mining are increasingly important and valuable.
Rare Earth Elements, REEs, also called rare earth metals or rare earth oxides, or lanthanides, are a set of 17 silvery-white soft heavy metals.
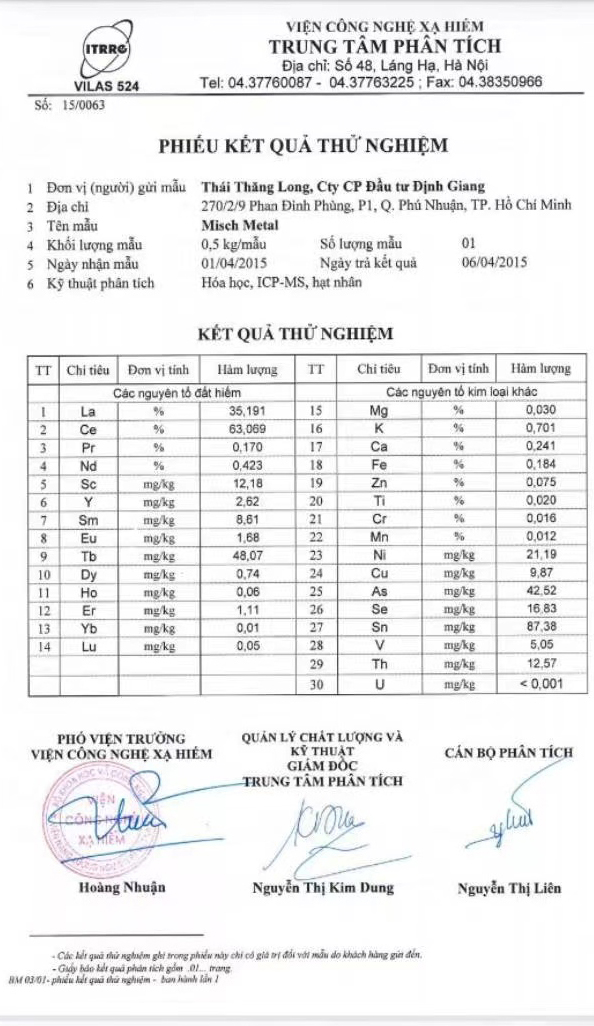
The 17 rare earth elements are: lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), lutetium (Lu), scandium (Sc), and yttrium (Y).
Scandium and yttrium are not part of the lanthanide family, but end users include them because they occur in the same mineral deposits as the lanthanides and have similar chemical properties.
Most of the elements are actually more abundant in Earth's crust than precious metals such as gold or platinum, but the properties of the 17 rare-earth elements are so similar to each other that extracting and purifying them requires sophisticated processes.
The term “rare earth” is a misnomer as rare earth metals are actually abundant in the Earth’s crust. However, they are rarely found in large, concentrated deposits on their own, but rather among other elements instead.
Rare earth elements are relatively plentiful in the earth’s crust, with cerium being the 25th most abundant element at 68 parts per million. This makes it as abundant as copper. Because of their geochemical properties, rare earth elements are typically dispersed. This means they are not often found in concentrated enough clusters to make them viable to mine. It was the scarcity of these minerals that led to them being called rare earths.
Rare Earths are a group of 15 elements in the periodic table known as the Lanthanide series, plus Yttrium. Rare earths are categorised into light elements (lanthanum to samarium) and heavy elements (europium to lutetium). The latter are less common and consequently more expensive.
Chemically, rare earths are strong reducing agents. Their compounds are generally ionic and they display high melting and boiling points. Rare earths are relatively soft when in their metallic state while those with a higher atomic number tend to be harder. Rare earths react with other metallic and non-metallic elements to form compounds each of which has specific chemical behaviours. This makes them indispensable and non-replaceable in many electronic, optical, magnetic, and catalytic applications. Rare earth compounds are commonly fluorescent under ultraviolet light, which can assist in their identification. Rare earths also react with water or diluted acid to produce hydrogen gas.
Rare Earths Key Attributes
Yttrium or Y (39) – is a non-lanthanide rare earth element used to produce superconductors, powerful pulsed lasers, cancer treatment drugs, rheumatoid arthritis medicines, and surgical supplies. Silvery metal in colour, it is also used in many popular consumer products such as televisions and camera lenses.
Lanthanum or La (57) – is a silver-white metal that is one of the most reactive rare earth elements. It is used in the manufacture of specialised optical glasses including infrared absorbing glass as well as camera and telescope lenses. It can also be used to help make steel more malleable. Other applications for lanthanum include wastewater treatment and petroleum refining.
Cerium or Ce (58) – is a silvery-white metal that easily oxidises in the air. It is the most abundant rare earth elements and has many uses including as a catalyst in catalytic converters in automotive exhaust systems to reduce emissions. It is also a key component for precision glass polishing. Cerium can also be used in iron, magnesium and aluminium alloys, magnets, certain types of electrodes, and carbon-arc lighting.
Praseodymium or Pr (59) – this soft, silvery metal was first used to create a yellow-orange stain for ceramics. Although it is still used to colour certain types of glasses and gemstones, praseodymium is primarily used in rare earth magnets. It can also be found in applications as diverse as creating high-strength metals found in aircraft engines and flint for starting fires.
Neodymium or Nd (60) – is a soft, silvery metal, neodymium that is used with praseodymium to create some of the strongest permanent magnets available in the world today. These magnets are found in most modern vehicles and aircraft as well as popular consumer electronics such as headphones, microphones, and computer discs. Neodymium is also used to make high-powered, infrared lasers for industrial and defence applications.
Promethium or Pm (61) – is the only naturally radioactive rare earth element. Virtually all promethium in the earth’s crust has long ago decayed into other elements. Today, it is largely artificially created, and used in watches, pacemakers, and a scientific research applications.
Samarium or Sm (62) – is a silvery metal that can be used in several vital ways including in very powerful magnets for transportation, defence, and commercial technologies. In conjunction with other compounds, it can also be used for intravenous radiation treatments to kill cancer cells. It is also used to help treat lung, prostate, breast, and some forms of bone cancer. Because it is a stable neutron absorber, samarium is also used to control the rods of nuclear reactors.
Europium or Eu (63) – is a hard metal used to create visible light in compact fluorescent bulbs and colour displays such as in LCD televisions. It is also used to make the special phosphors marks on the Euro notes to help prevent counterfeiting.
Gadolinium or Gd (64) – has particular properties that make it especially suitable for shielding in nuclear reactors and neutron radiography. It is also used to help target tumours in neuron therapy as well as enhance magnetic resonance imaging (MRI). X-rays and bone density tests also use gadolinium, making this rare earth element a major contributor to modern health care solutions.
Terbium or Tb (65) – is a silvery rare earth metal that is so soft it can be cut with a knife. Terbium is often used in compact fluorescent lighting, colour displays, and as an additive to permanent rare earth magnets so they can function better under higher temperatures. It can also be found in fuel cells designed to operate at elevated temperatures and some electronic devices as well as naval sonar systems. Terbium in its alloy form has the highest magnetostriction of any such substance. Because of its magnetisation, in its alloy form it is easy to change its shape, making it vital component of Terfenol-D which is used in many defence and commercial technologies.
Dysprosium or Dy (66) – is a soft, silver metal with one of the highest magnetic strengths of all of the rare earths, matched only by holmium. Dysprosium is often added to permanent rare earth magnets to help them operate more efficiently at higher temperatures. Lasers and commercial lighting use dysprosium as do hard computer disks and other electronics which require certain magnetic properties. Dysprosium can also be used in nuclear reactors and modern, energy-efficient vehicles.
Holmium or Ho (67) – has incredible magnetic properties. In fact, some of the strongest artificially created magnetic fields are the result of magnetic flux concentrators made with holmium alloys. As well as providing colouring to cubic zirconia and glass, holmium can also be used in nuclear control rods and microwave equipment.
Erbium or Er (68) – is widely used in nuclear applications such as neutron-absorbing control rods. It is a key component of high-performance fibre optic communications systems. It can also be used to provide glass and other materials a pink colour for aesthetic as well as specific industrial purposes. Erbium is also used to help create lasers, including those for specific medical applications.
Thulium or Tm (69) – is a silvery-grey metal and one of the least abundant rare earths. Its isotopes are widely used as the radiation device in portable X-rays which makes thulium a highly useful material. Thulium is also a component of highly efficient lasers which are used in the defence, medical, and meteorology sectors.
Ytterbium or Yb (70) – has several important uses in health care including in certain cancer treatments. Ytterbium can also enhance stainless steel as well as help monitor the effects of earthquakes and explosions on the ground.
Lutetium or Lu (71) – is the last of the rare earth elements (in order of their atomic number) and has several interesting uses. For example, lutetium isotopes can help reveal the age of ancient items such as meteorites. It also has applications related to petroleum refining and positron emission tomography. Experimentally, lutetium isotopes have also been used to target certain types of tumours.
Use of Rare Earth Elements
Most rare earth elements find their uses as catalysts and magnets in traditional and low-carbon technologies. Other important uses of rare earth elements are in the production of special metal alloys, glass, and high-performance electronics. Alloys of neodymium (Nd) and samarium (Sm) can be used to create strong magnets that withstand high temperatures, making them ideal for a wide variety of mission critical electronics and defense applications.
REE Usage % of 2019 Rare Earth Demand
Permanent Magnets 38%
Catalysts 23%
Glass Polishing Powder and Additives 13%
Battery Alloys 9%
Metallurgy and Alloys 8%
Ceramics, Pigments and Glazes 5%
Phosphors 3%
Other 4%
REE Current Applications
| Yttrium | Phosphors , ceramics, metal alloys |
| Lanthanum | Batteries, catalysts for petroleum refining |
| Cerium | Autocatalysts, Chemical Catalyst, glass polishing, metal alloys |
| Praseodymium | High power magnets, yellow ceramic pigment, Autocat |
| Neodymium | High power magnets |
| Promethium | Beta radiation source |
| Samarium | High temperature magnets, |
| Europium | fluorescent lighting |
| Gadolinium | Magnetic resonance imaging contrast agent, nuclear reactor rods |
| Terbium | Phosphors for lighting, high power high temperature magnets |
| Dysprosium | High power high temperature magnets, lasers |
| Holmium | Highest power magnets in existence |
| Erbium | Lasers, glass colourant |
| Thulium | Ceramic magnetic materials which are still under development |
| Ytterbium | Fibre optic technology, solar panels |
| Lutetium | PET scanners |
REE Compound Applications
|
| |
|---|---|
| Catalysts | Petroleum refining |
| Chemical processing | |
| Catalytic converters | |
| Diesel additives | |
| Industrial pollution scrubbers | |
| Glass | Polishing compounds |
| Optical glass | |
| UV resistant glass | |
| X-ray imaging | |
| Thermal control mirrors | |
| Colourisers / deodarisers | |
| Metal Alloys | Hydrogen storage NiMH batteries; fuel cells |
| Steel | |
| Lighter flints | |
| Aluminum / magnesium | |
| Cast iron | |
| Superalloys | |
| Electronics | Display phosphors LCD, PDP, CRT |
| Medical imaging phosphors | |
| Lasers | |
| Fibre optics | |
| Optical temperature sensors | |
| Ceramics | Capacitors |
| Sensors | |
| Colourants | |
| Scintillators | |
| Magnets | Motors |
| Disk drives and disk drive motors | |
| Power generation | |
| Actuators | |
| Microphones and speakers | |
| MRI | |
| Anti-lock brake systems | |
| Automotive parts | |
| Communication systems | |
| Electric drive and propulsion | |
| Frictionless bearings | |
| Magnetic storage disks | |
| Microwave power tubes | |
| Magnetic refrigeration | |
| Magnetostrictive alloys |
Rare Earths For Human Daily Life
REEs are the backbone to many of the devices we use on a daily basis, as well as the technologies that are already contributing to the health of our planet.
RARE EARTH ENHANCE ENVIRONMENTAL PROTECTION AND REDUCE GREENHOUSE GAS EMISSIONS
Global warming due to greenhouse gas emissions is a concern for us all. Rare earths already play a vital role in the reduction of greenhouse gas emissions.
Many scientists believe that global warming is caused by a human-driven increase in greenhouse gases in the earth’s atmosphere. With our society becoming more aware of the part we have to play in addressing global warming, governments of today are now legislating higher environmental and lower emission standards in both domestic and industrial settings.
Rare earths are playing a pivotal role in greenhouse gas reduction through their unique application in automotive catalytic converters, hybrid vehicles, wind turbines, and energy efficient compact fluorescent light bulbs.
RARE EARTH ENABLE DIGITAL TECHNOLOGY
The digital era continues to gather pace; broadband access, digital television, digital cameras, and digital music are around us at home and on the move – rare earths are enablers of this technology and its miniaturisation.
New materials and novel applications enable companies to produce more efficient, higher performance materials, which meet the demand for faster, smaller and lighter products.
RARE EARTH IMPROVE ENERGY EFFICIENCY
Increased population and economic growth leads to greater demand of the world’s energy, which means increased use of our limited fossil fuel reserves. Rare earths are already playing a vital role in conservation of these reserves, and are likely to play an even larger role in taking us forward to the hydrogen economy.
The world’s fossil fuels are limited, however with the billions of dollars invested in the global oil and gas infrastructure, it is important we use these reserves efficiently.
Rare earths are supporting the uptake of energy efficient initiatives through their unique physical and chemical properties, which allow them to protect the environment (by lowering energy consumption), and improve lifestyles (through energy efficient alternatives that save money without sacrificing comfort and reliability).
RARE EARTH PRODUCTS
- Wind turbines. NdFeB magnet enables direct drive wind turbines technology providing better electrical yield, reduced maintenance and improved reliability (no gear box).
- Automotive catalyst. Cerium-based new technology enables Lean NOx Trap – a very compact catalytic system that allows diesel compact cars to match with NOx emission regulation
- Hybrid, plug hybrid and electrical vehicles. A fast growing segment which contributes to controlling pollution at its point of emission
- Vehicles in general. NdFeB magnet enables substantial weight reduction contributing to overall energy savings and reduction of CO2 emission
Global Supply of REEs
In the rare earth industry, China’s dominance has been no accident. Years of research and industrial policy helped the nation develop a superior position in the market, and now the country has the ability to control production and the global availability of these valuable metals.
This tight control of the supply of these important metals has the world searching for their own supplies. With the start of mining operations in other countries, China’s share of global production has fallen from 92% in 2010 to 58%< in 2020. However, China has a strong foothold in the supply chain and produced 85% of the world’s refined rare earths in 2020.
China awards production quotas to only six state-run companies:
- China Minmetals Rare Earth Co
- Chinalco Rare Earth & Metals Co
- Guangdong Rising Nonferrous
- China Northern Rare Earth Group
- China Southern Rare Earth Group
- Xiamen Tungsten
In an effort to overcome China’s near-monopoly on the supply of rare-earth elements, Japan and Vietnam have launched a joint research centre in Hanoi to improve extraction and processing of the materials.
Rare-earth elements include scandium, yttrium and the 15 lanthanides found towards the bottom of the periodic table. Their unique optical and magnetic properties are used in various high-tech applications, such as motors, catalysts, light-emitting diodes and batteries.
Global Reserves of Rare Earth Minerals
China tops the list for mine production and reserves of rare earth elements, with 44 million tons in reserves and 140,000 tons of annual mine production.
While Vietnam and Brazil have the second and third most reserves of rare earth metals with 22 million tons in reserves and 21 million tons, respectively, their mine production is among the lowest of all the countries at only 1,000 tons per year each.
| Country | Mine Production 2020 | Reserves | % of Total Reserves |
|---|---|---|---|
| China | 140,000 | 44,000,000 | 38.0% |
| Vietnam | 1,000 | 22,000,000 | 19.0% |
| Brazil | 1,000 | 21,000,000 | 18.1% |
| Russia | 2,700 | 12,000,000 | 10.4% |
| India | 3,000 | 6,900,000 | 6.0% |
| Australia | 17,000 | 4,100,000 | 3.5% |
| United States | 38,000 | 1,500,000 | 1.3% |
| Greenland | - | 1,500,000 | 1.3% |
| Tanzania | - | 890,000 | 0.8% |
| Canada | - | 830,000 | 0.7% |
| South Africa | - | 790,000 | 0.7% |
| Other Countries | 100 | 310,000 | 0.3% |
| Burma | 30,000 | N/A | N/A |
| Madagascar | 8,000 | N/A | N/A |
| Thailand | 2,000 | N/A | N/A |
| Burundi | 500 | N/A | N/A |
| World Total | 243,300 | 115,820,000 | 100% |
Vietnam REEs Research - Center for Rare Earth Research and Technology Transfer in Dan Phuong District, Hanoi
The joint research project between Vietnam and Japan is of special significance as Vietnam has great potential for exploring rare earth mines but the industry is in its infancy. The center is the first facility in Vietnam applying Japanese technology to separate rare earths from ore before they are shipped to Japan.
DOCUMENTS
- United States Geological Society (USGS) reveals the countries with the largest known reserves of rare earth elements (REEs).
- Vietnam Office Of Quality Acceptance
- Loading...









